External Control Arms: The Way to Go?
The growing implementation of Single-Arm Trials (SATs) with External Control Arms (ECAs) has been the subject of heated debate across Europe. Some advocate its use when traditional Randomized Control Trials (RCTs) are unfeasible for a variety of reasons, while others fear that using ECAs is a means to accelerate timelines and limit costs at the expense of robust quality of evidence.
At ISPOR Europe 2022, Catia Proenca, Director of Real World Solutions at Alira Health, provided an overview of the regulatory and HTA use of ECAs, supported by case studies and inputs from patient-representatives about their perspective on usage of ECAs.
What Are External Control Arms?
In a RCT, patients are randomly split into experimental arms (those who receive the new therapy) and control arms (those who receive a placebo and/or the standard of care). The European Medicines Agency (EMA) defines an ECA as an external group of patients who are not part of the same study receiving the intervention and are therefore not composed of the same population. Using an ECA, a sponsor relies on prior clinical trial data or Real World Data (RWD) that already exists; either historical data from a group of patients treated at an earlier time, or contemporaneous, which refers to a group treated during the same time period as the clinical trial but in a different setting
ECAs can be used in multiple contexts:
- In the case of rare diseases: when there are too few people with a specific disease to create sufficient experimental and control arms. It’s important to note that many diseases are becoming rarer; for example, with many cancers, researchers can narrow a patient’s specific disease to a few genetic mutations, with the effect of making that cancer limited to fewer patients.
- When it would be unethical to randomize: if providing a placebo would be unduly unfair to those patients (lack of equipoise), or when there is not a comparable standard of care treatment.
- When an ECA could complement an RCT reducing the sample size of the control arm
ECAs Increasingly Used to Support Regulatory and HTA Decision-Making
The data shows a transforming global ecosystem. In the past decade, there has been a steady increase in the submission of dossiers to Health Authorities and Health Technology Assessment (HTAs) containing Real World Evidence (RWE) and ECAs to support the demonstration of efficacy. The number of clinical trials using SATs registered on Clinicaltrials.gov between 2014 and 2021 went from 15 to 184. Key stakeholders (U.S. Food and Drug Administration and EMA) have acknowledged and encouraged the use of RWD/E (e.g., 21st Century Cures Act). Also, HTA bodies in some countries are publishing guidance documents setting out the recommendations for RWD study design and execution, including for ECA design.
What is the HTA Perspective on the Acceptance of SATs with ECAs?
To discover what HTAs think about SATs with ECAs, Alira Health conducted a study. The study approach was to:
- Perform a targeted literature review to identify technologies that were submitted with SAT and ECA.
- Review the HTA appraisal reports of the selected technologies to assess how HTAs evaluated ECA data in their appraisals.
- Interview HTA/payer experts in EU4+UK to gather insights about the acceptance and criteria for ECA design.
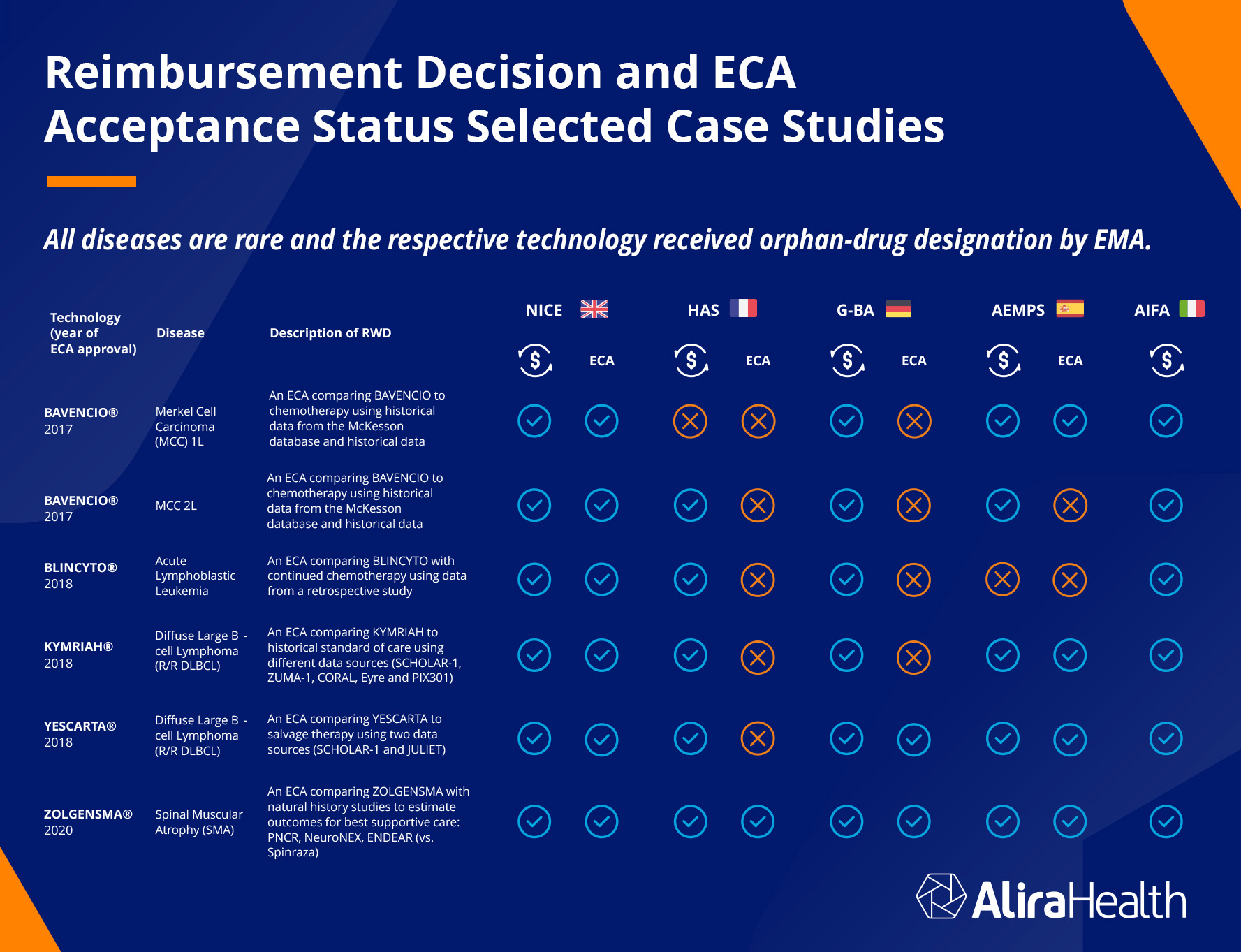
The technologies identified were all targeting rare diseases, and the respective technologies all received orphan-drug designation by EMA. The reimbursement decisions and ECA acceptance status were examined across five countries: the United Kingdom, France, Germany, Spain, and Italy (note that Italy’s reporting did not include ECA acceptance). The chart below shows the results.
The UK reimbursed all technologies and accepted all the ECAs. France failed to reimburse for one technology, and rejected all ECAs but one. Germany reimbursed for all and rejected four out of six ECA. Germany’s HTA will not accept ECAs readily because their standards are very high: if the treatment group does not do dramatically better or see dramatic improvement over the control group, the HTA will not approve. Many of these technologies only deliver incremental improvement, which still can mean a lot to a patient.
Italy reimbursed for all, and Spain reimbursed for six and accepted four ECAs. The majority of reimbursements (28 out of 30) were made, and of the ECAs submitted for approval, 13 out of 24 were approved.
Recommendations for Successful Use of ECAs in SATs
The study concluded that successful ECAs in SATs must follow a robust study design and high RWD quality. Companies developing drugs or devices should follow these parameters as much as possible when designing clinical trials that will use an ECA, in order to be accepted by the HTAs in each country.
- Pre-specify statistical analyses plan, list confounders
- Match the patient characteristics and endpoint definition of ECA with the SAT
- Include objective endpoints that can accurately be tracked with RWD
- Use patient-level data which is preferred over aggregated as it allows for more robust analyses
- Demonstrate the quality of the RWD source and criteria or selected source
- Employ adequate statistical analyses to limit bias such as propensity-score weighting methods
- Implement pre-specified sensitivity analysis to test assumptions
Need help designing your clinical trial?
Contact us to learn how we can help you design your clinical trials using single-arm trials with external control arms.
Subscribe to our newsletter for the latest news, events, and thought leadership