Home » Education hub » WHO Model Lists of Essential Medicines: What You Need to Know
WHO Model Lists of Essential Medicines: What You Need to Know
WHO Model Lists of Essential Medicines: What You Need to Know
In 1977, the World Health Organization (WHO) created the concept of essential medicines by launching the first Essential Medicines List (EML). In this article, our Market Access experts share the importance of the EML for pharma companies and four best practices for a successful application, based on our extensive experience in interactions with the WHO.
The EML: What It Is and Why It Matters
According to the WHO, “Essential medicines are the medications considered to be most effective and safe to meet the most important health needs in a country.“ The WHO selects these medicines for the EML based on issues such as their importance to public health, their clinical efficacy and safety, and their cost-effectiveness compared to other available treatments, as well as disease prevalence. The EML is part of the WHO’s health equity mission, with the goal of providing patients with consistent access to these vital drugs when needed.
The WHO EML is an important part of many national healthcare policies, as well as many international organizations, non-governmental bodies, and non-profit suppliers. Pharma companies seek to include their drugs on the EML to improve patient access worldwide.
Best Practices for Your EML Application
1. Include all the necessary elements in your WHO EML application (submission dossier):
- General items: in this section, you include information about the medicine(s) proposed for inclusion, i.e., International Nonproprietary Names, Anatomical Therapeutic Chemical code, and treatment details.
- Public health relevance must be demonstrated by providing epidemiological information on disease burden, target population(s), and alternative medicines already included on the EML for the proposed indication(s), if any.
- Evidence appraisal and synthesis: this section represents the cornerstone of the dossier in which you provide the background on the clinical evidence of the medicine and the added value vs. its comparators. It includes the summary of available evidence for comparative effectiveness and safety, cost, and cost-effectiveness. Whenever possible, you should present systematic literature reviews, meta-analyses, and detailed methodology for evidence search, as well as appraisal of quality and a compelling summary of results.
- Regulatory information: a summary of regulatory status and market availability of the medicine, as well as availability of pharmacopeial standards.
- References: a comprehensive reference list and in-text citations.
Based on our experience with the WHO, you need to develop all these elements to complete a successful application. The more information you provide, the better the expected outcome. The application must evaluate data for both adults and children (when applicable to the proposed medicine).
2. Focus on timelines
The EML is updated every two years but does not follow a strict calendar. The most recent update took place in 2023. Currently, there are no fixed dates for new requests in 2024 to update the list in 2025. Make sure you closely follow the calendar updates and do not miss the application slots.
Because the submission dossier must follow a specific structure and you may need to build it from scratch if you don’t have all the necessary information, you need to plan on a substantial time investment.
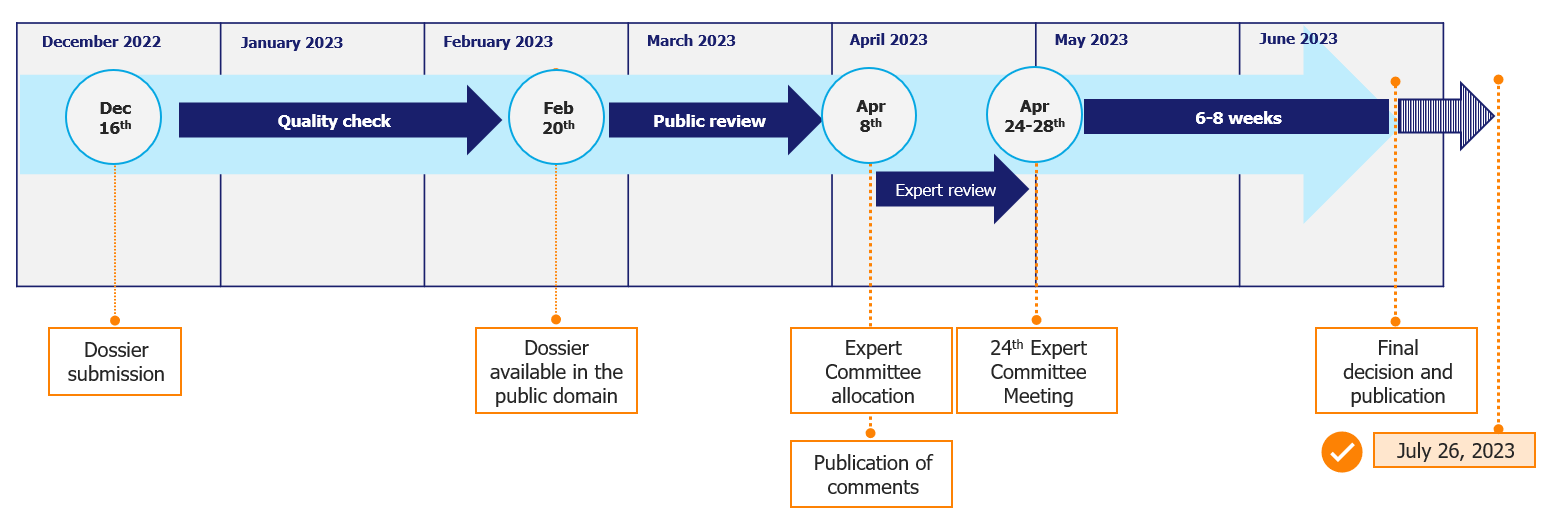
According to the WHO, the publication of the EML 2023 could be expected to take place from 6 to 8 weeks after the Expert Committee Meeting. However, these timelines could be more fluid, since it took 13 weeks in 2021 and 11 weeks in 2019.
3. Focus on inclusion criteria
Comparative cost and cost-effectiveness are the backbone of the application for inclusion. These are the critical elements upon which the WHO’s expert committee judgments are made. Evidence reviews must be based on systematic literature reports and/or meta-analyses, either published or provided by you together with a comprehensive methodological approach.
The submission should consider not only the clinical evidence but the overall financial impact on health systems of making the medicine available. You should provide general guidance on whether the intervention provides good value for money compared to alternatives.
4. Provide reliable and reputable endorsements
External endorsement to your submission is paramount to success. Ensure that the company has a targeted strategy to bring KOLs onboard that can advocate for the medicine; plan this in advance because it takes time and effort. You can add expert letters from medical societies and non-profit organizations as appendices to the dossier and as comments to submissions before the appraisal committee meeting takes place. These organizations can even submit the dossier on your behalf, assuming authorship.
Subscribe to our newsletter for the latest news, events, and thought leadership
